If you are reading this you might be asking yourself, “Self, how do I capture meaningful data with my infrared camera?” Or, “Self, do I know enough of the basics of thermography in order to perform a thermal analysis?” Or, “Self, why in the world are you speaking in the third person?” In the world of thermography there are many considerations that must be made when taking and analyzing the images captured but there is one piece of science that is most important in obtaining meaningful results. This is especially true if your goal is to take even remotely accurate temperature readings using your IR camera. This little nugget of thermographic gold is:
Energy Absorbed + Energy Reflected + Energy Transmitted = Total Energy Observed from a Body
OR
α + ρ + τ = 1
where:
α= Ratio of Energy Absorbed by a Body to Total Energy Observed from a Body
ρ = Ratio of Energy Reflected by a Body to Total Energy Observed from a Body
τ = Ratio of Energy Transmitted by a Body to Total Energy Observed from a Body
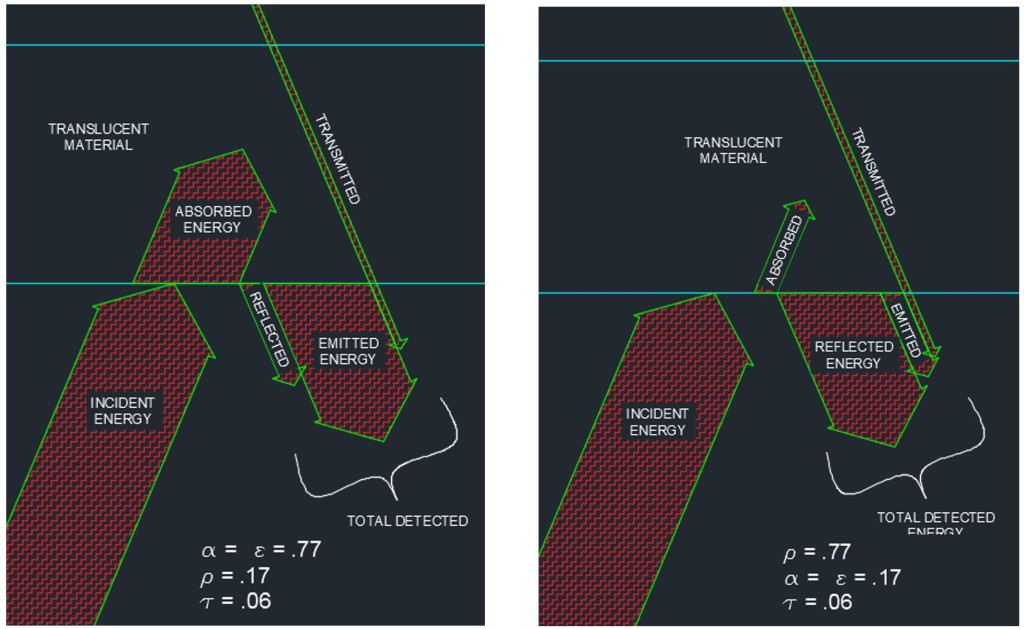
Translucent Material, Highly Absorptive vs. Translucent Material, Highly Reflective
Bear with me while I lay out a couple of necessary concepts. For most opaque materials, the transmitted energy is equal to zero. Even my children can grasp this concept when I explain to them that we don’t typically feel the warmth of the sun directly while standing in the shade. We feel the temperature of the air around us. The infrared energy is not able to penetrate most opaque objects so this concept is somewhat intuitive. The implication of this fact is that for most objects which appear in an IR image, the transmittance can be taken to be zero. Additionally, in the mid-1800’s, Gustav Kirchoff gave us a relationship that came to be known as Kirchoff’s Law of Thermal Radiation. I will significantly simplify Kirchoff by telling you that his Law of Thermal Radiation states that, for a body at thermodynamic equilibrium (constant temperature), the absorptivity is equal to the emissivity (α = ε).

Pasta Sauce on Stove – Delicious!
Simply put, whatever thermal energy the object absorbs, it also radiates that same amount back to the atmosphere. If the absorptivity and emissivity were different the object would be in the midst of a temperature change. Think of a pot of tomato sauce with the lid on left to simmer on a stove. The temperature of the pot is hot enough to burn you if you touch it but it has reached a constant temperature and is no longer getting any hotter. The energy absorbed from the burner by the pot (absorptivity) is equal to the energy emitted to the atmosphere by the pot (emissivity).
So, we can simplify and restate our previously identified relationship to be:
ε + ρ =1
where:
ε = Ratio of Energy Emitted by a Body to Total Energy Observed from a Body
ρ = Ratio of Energy Reflected by a Body to Total Energy Observed from a Body
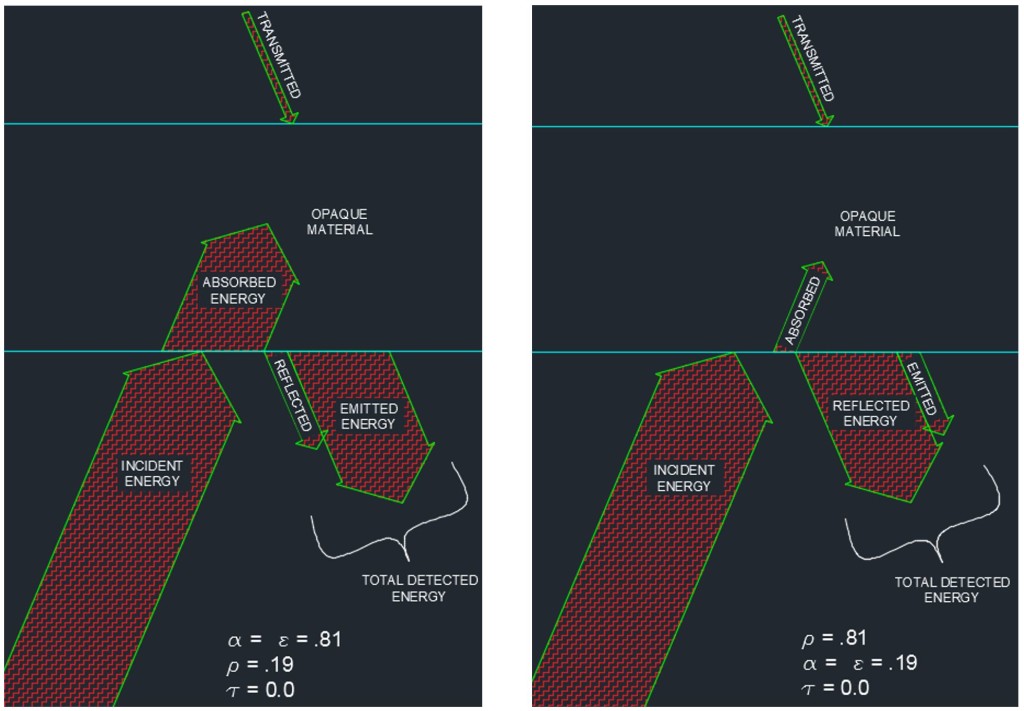
Opaque Material, Highly Absorptive vs. Opaque Material, Highly Reflective
How about a couple of real life images to illustrate this?We can now see an inverse relationship between the thermal reflectance and the thermal emissivity of an object. The more reflective something is, less of the energy that we see come from its surface is actually emitted by that object.
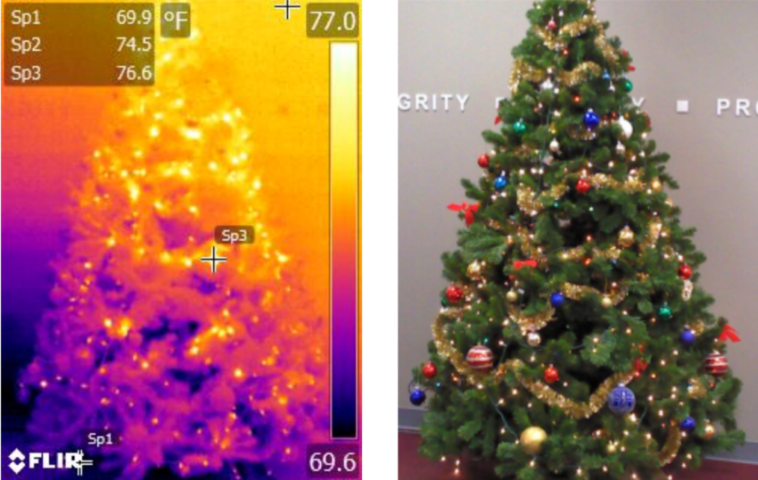
IR & Visual Images of Christmas Tree
In the image above we see a traditional Christmas tree and the corresponding thermal image. In this image we can clearly see a thermal gradient from floor to ceiling as is to be expected. We also, unsurprisingly, see bright spots where the lights are emitting additional heat being picked up by the IR camera. This is a good example of looking at the thermal energy being emitted by what are mostly opaque, highly emissive objects. Now let’s take a closer look…
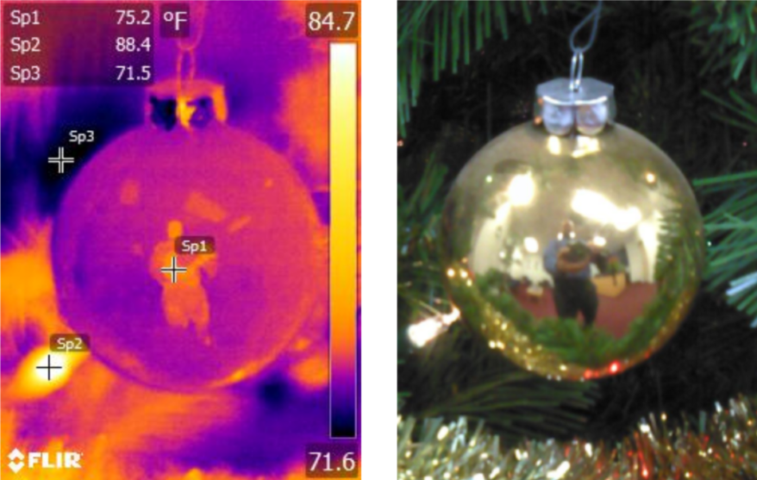
IR and Visual Images of Ornament
In the above image we are looking at one of the ornaments on the tree shown in the previous image. We can see that it is highly reflective in the visual spectrum. When we look at the IR image, sure enough, there is a reflection of the person taking the picture here as well. The temperature shown on the ornament is going to be rather inaccurate because it is actually measuring a portion of the photographer’s heat energy. This image demonstrates the difficulty we can experience when taking IR images without regard to the nature of the object we are documenting. We must have an understanding of what the material and finish is of the object we are analyzing in the IR images.
What is the bottom line to all of this? Just because your IR camera says that the temperature is 106.5oF, doesn’t mean that it is. When we look at an object with a highly reflective surface we may be picking up a portion of the IR energy of other sources in the area. If we are looking at something that may be translucent to IR wavelengths, we may be seeing the IR energy of objects behind what we are attempting to measure. In order to obtain meaningful results from our IR images we must account for these factors (and many, many others – but those are for later posts!). Be sure to stop by often to stay up to date on our series of building science posts. Some of our future topics will include: The Primary Concerns of Thermal Image Capture – FORD, Accounting for Environment Interference in Thermal Images, Envelope U-value and Moisture Migration, The Wonder of Thermal Hysteresis, and much, much more! If there are other topics that you would like to see us cover, feel free to contact us and let us know.

